-
Product
Businessmap SOFTWARE PLATFORM (formerly Kanbanize)
- Demo library Watch on-demand demos
- Smart Canvases Plan & ideate with smart canvases
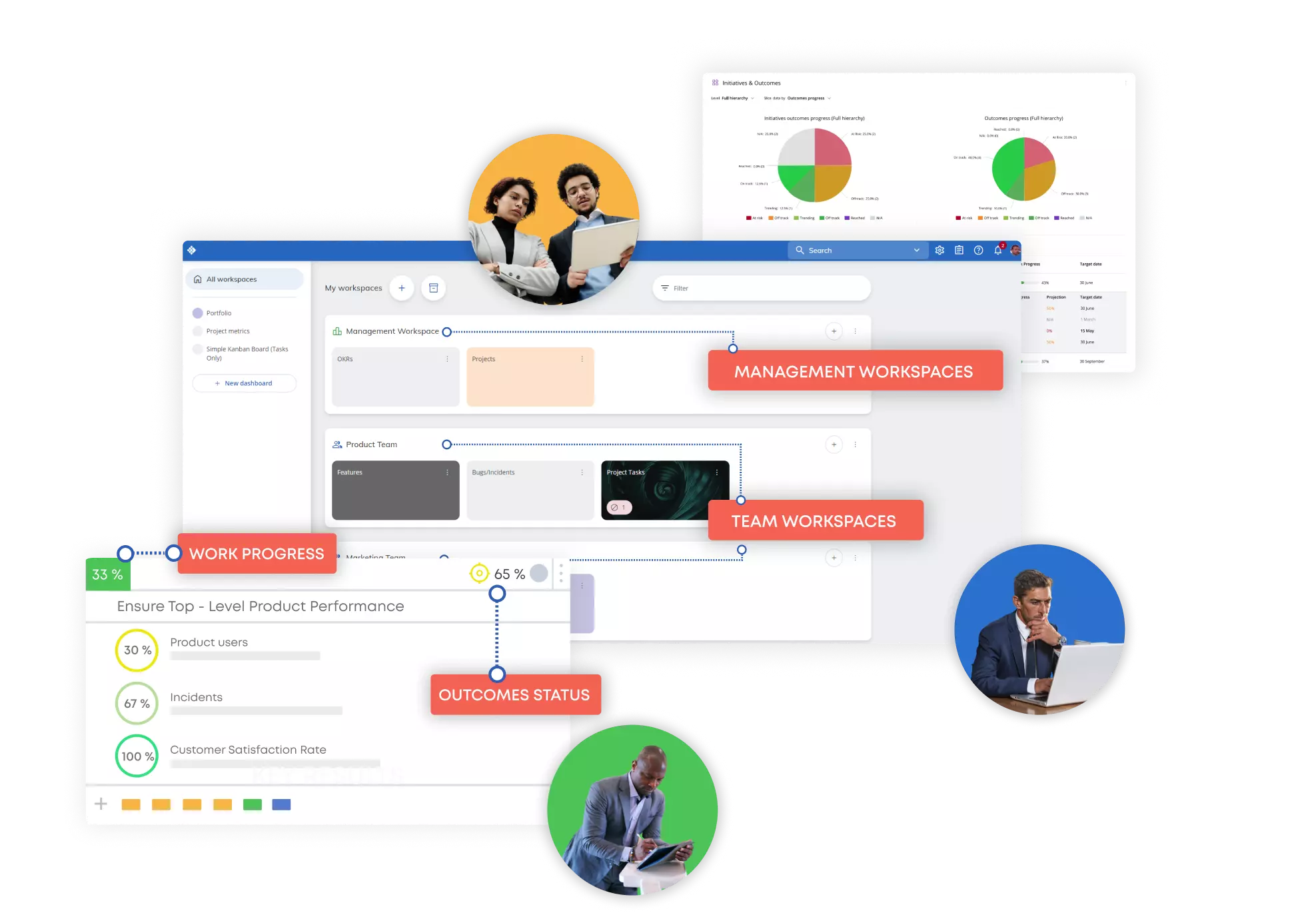
- Portfolio Workspaces Increase visibility across projects, and connect planning with execution
- Management Dashboards Monitor business objectives, understand risks, and track the most important performance metrics
- AI Tools Discover the power of AI tools to transform work management and improve efficiency
- Kanban Boards Keep track of tasks and get accurate status reports in real-time
- See all functionalities
-
Solutions
BY USE CASE
- Project and Portfolio Management Visualize ALL projects and deliver faster
- Agile Project Management Embrace Agile project planning across the entire organization
- OKRs Project Management Align your projects and connect work to business outcomes
- Kanban Project Management Visualize and improve any work process
BY INDUSTRY- Mechanical Engineering Gain unmatched visibility and streamline engineering processes
- Hardware Product Development Enable Lean/Agile workflows across your product development lifecycle
- Oil & Gas Project Management Align on goals and manage workflow complexity in the Oil & Gas industry
- Pharma & Biotech Project Management Eliminate waste across the R&D lifecycle & gain clarity over clinical trials workflows
- Partners
- Pricing
- Enterprise
-
Resources
Helpful Resources
- See full product demo Align teams on goals and gain work visibility
- Take a guided tour Experience Businessmap in a production-like environment
- Businessmap Academy Dive into Lean/Agile with dedicated courses
- Read Case Studies Read how companies align strategy with execution
- Businessmap Blog Discover the latest Businessmap news, get helpful content and actionable tips
- Knowledge Base Configure our software platform and keep track of new product updates
- Kanban Simulation Get to know Kanban in a simulation environment
- Watch Customer Stories
- A Guide To Enterprise Agility For Project Organizations
- The Businessmap Way Explore our implementation services for a smooth organizational transformation