Why Does the Traditional Operating Model Give Way to Agile in Pharma?
Pharmaceuticals is known as a heavily regulated and specialized industry. The strict quality requirements and processes, combined with long and cost-intensive drug development processes, might sound utterly incompatible with the Agile methodologies and principles. Nevertheless, Agile in Pharma is on the rise.
Many pharmaceuticals, health services, and medical device companies have recognized that the new dynamic market conditions call for a change in their operations models. Challenges like increased complexity in the customer landscape and micro-targeted research with lower ROI potential are forcing companies to search for new ways to increase speed to market, lower development costs, and increase overall operational efficiency. Pharmaceutical companies are already undergoing digital transformations as a result of the growing demand.
The Burning Challenges in the Pharma Industry and
Why Is Organizational Agility the Key to a Successful Digital Transformation?
Further on, the lack of a direct relationship with their patients has left pharmaceuticals with one blind eye, relying on clinical data and staying unaware of the customers` expectations and needs. To successfully overcome this and other constraints, the Pharma players are turning to Agile practices. The goal is to support an operational evolution favoring experimentation, customer focus, and collaboration.
How is Agile Transforming the Pharma Industry?
Increased Process Agility
Many pharmaceutical companies seek to transform their R&D departments into a source of competitive advantage. To accelerate their development times, some introduce daily progress discussions. Others transfer a big part of the decision-making to the team meetings to shorten approval chains and eliminate progress blockers.
McKinsey research data shows that in one year, a pharmaceutical company was able to double its R&D capacity without adding new resources. To achieve this, they combined the two approaches and extended Agile working to more than a dozen departments.
Accelerated Drug Development
New R&D models, like "Virtualized R&D," are also on the rise. Latest reports suggest that AI investments in drug development and design are 4.5 times higher in 2020 compared to other industries and previous years (source: Stanford HAI).
Conducting multiple tests to prove different statements is at the core of drug development. However, this process takes time and resources before approving or discarding a hypothesis and releasing a product on the market. Combined with Agile practices, AI helps pharma companies discover knowledge faster and improves the time to market for drug development. This is done by reducing the batch size of experiment-based work so data collection and analysis can be done faster.
With virtualized R&D, pharma companies can access specific expertise while also minimizing the buildup of infrastructure through collaboration with external partners. In extreme cases, the complete lab or clinical work is executed externally. Diversifying their portfolios through more external collaborations and accelerating the drug-development process makes rapid decision-making a critical success factor.
Cross-Functional Team Collaboration & Breaking Down Silos
Another Agile approach supporting pharma companies to keep up with the industry's new pace is the cross-functional team structure. Breaking the rigid silos and forming cross-functional working groups increases organizational agility, transparency, and employee engagement. Furthermore, the smaller groups are more flexible to iterate faster and accelerate their overall operational efficiency.
By reconfiguring brand responsibilities, a global pharmaceutical company reduced the time required to develop and implement a brand strategy from more than two years to 90 days. Before introducing the changes, three teams with over 40 members for each brand were responsible for the brand strategy. The cross-functional set-up reduced the team size to 8-12 members. To further accelerate the process and get to the results mentioned above, the cross-functional squads started working with MVPs and initiated business planning and budgeting changes.

Active Customer-Collaboration
To cover the blind spot on the customer side, some pharmaceutical companies are launching customer-collaboration initiatives with patients and physicians. Working with those stakeholder groups, they create prototypes for “beyond the pill” solutions and more customer-centric launch strategies. Through the collaboration, the companies gather new insights and launch continuous improvement initiatives for their products, going beyond R&D.
Most Common Challenges When Implementing Agile in Pharma
Implementing Agile practices in pharmaceutical companies comes with a fair number of challenges.
-
Rigid operations. Being a research-intensive industry, many of the staff tend to be highly specialized and used to sequential and rigid ways of working. The overall company culture also focuses on stability, pre-defined work, and strong process orientation.
-
Change resistance. “Being or doing Agile” goes beyond merely introducing daily stand-up meetings and MVPs. It requires a general mind-shift, more openness to change, and flexibility in the way of working. Creating dedicated initiatives that stimulate a cultural shift towards experimentation and cross-divisional collaboration can help minimize the risk of resistance.
For example, Pfizer has managed to develop a culture of experimentation through a “Dare to Try” program - a mix between tools, training, and a network of supporters.
-
Fit for purpose cross-functional teams. Defining a cross-functional team may tend to sound like an easy task. However, for the team to deliver up to the expectations, it must be "fit for purpose." Who must be on the team and who does not have to be included is a strategic decision that will define the newly formed group's overall effectiveness.
-
Processes restructuring. Which processes should be redesigned may vary from company to company. However, the focus should be on minimizing hand-offs to ensure a smooth flow of work, incorporating input from internal and external stakeholders, and creating MVPs to collect feedback early in the process.
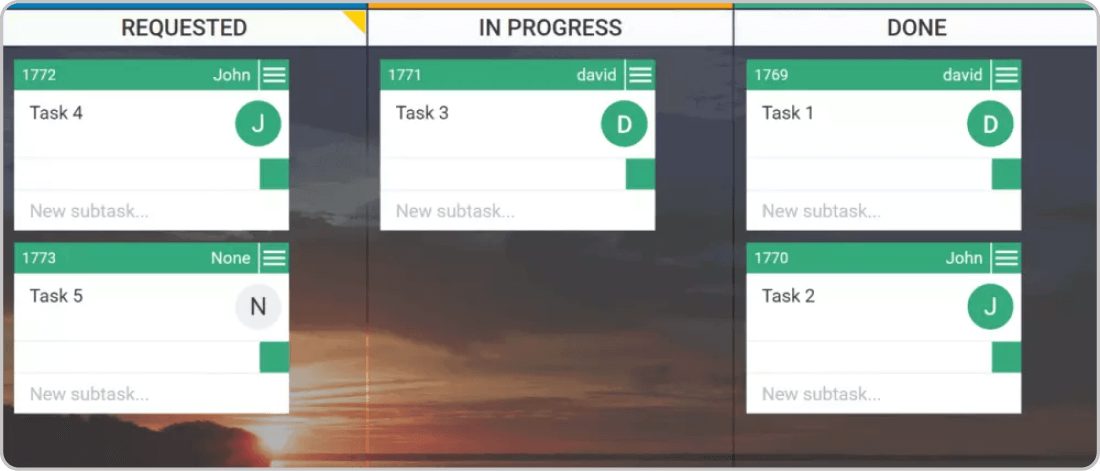
A good practice that can help you build a stable workflow and enhance communication during the Agile implementation is to visualize the work and the company's different workflows. A popular approach for work visualization is using a Kanban board. Having a visual overview of the processes helps you better understand your operations and quickly identify weak spots.

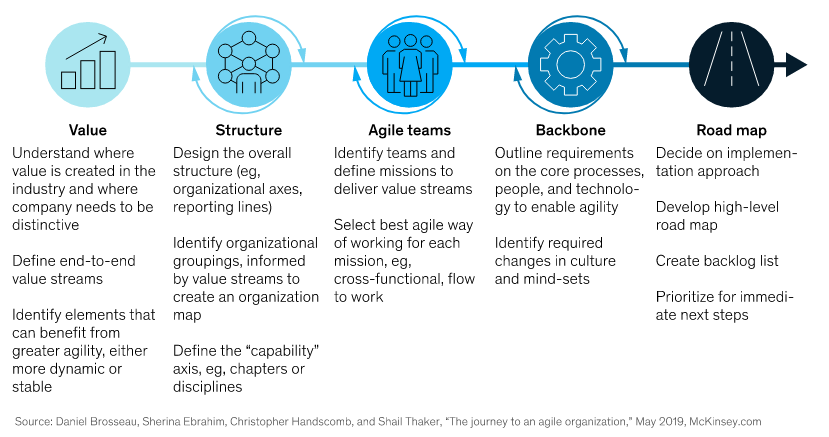
Success Factors for Agile Transformation in Pharmaceutical Companies
Initiating an Agile transformation needs more than knowing and implementing the Agile practices that have worked in other industries. McKinsey has identified several critical factors for a successful Agile transformation in pharmaceutical companies:
-
Clear and shared vision. Launching a transformation initiative without a clear vision of its goals or the expected changes will inevitably cause a lot of distress and confusion in the company. A shared understanding of why the transformation is needed and how the new way of working will look is required so management can align everyone around this vision.
-
Change in the culture. Evangelizing the Agile principles without translating them into every-day actions can be a stumbling stone on the path to agility. A dedicated team that manages and communicates the agile-inspired changes are crucial in supporting the overall cultural shift.
-
Building new skillset. The new ways of working will also require new skills. It is advisable to support the development of expertise in analytics, clinical trial design, and vendor management to tap fully into Agile's potential.
-
Measure and course correct. The success of your Agile transformation depends on setting and monitoring KPIs that will play the role of your transformation compass. Using digital tools for a real-time overview of the metrics will provide data on your progress and will help you keep your transformation efforts on track.

Photo credits: McKinsey
Ready to see Businessmap in action?
In Summary
Agility is defined as an organization's ability to effectively satisfy its target market through rapid experimentation and timely course adjustment. Agility in the pharma industry would translate this into shorter development cycles, more external collaboration with partners and key stakeholders, and faster reallocation of resources to reduce time to market.
To accelerate innovation, shorten time-to-market and increase operational efficiency, pharmaceutical companies are adopting Agile practices, leading to significant changes in the way they operate:
-
Reshaped R&D departments. Disrupting the traditional Waterfall-oriented operation model with more Agile practices, the companies are introducing daily stand-ups, MVPs, cross-functional teams, and collaboration initiatives.
-
Streamlined decision-making process. Transferring a significant part of the decisions to team-level meetings helps remove bottlenecks, avoid miscommunication, and improve the flow of work.
-
More internal and external feedback and collaboration. Moving away from blindly relying on clinical data, companies are opening up to cross-divisional and customer collaboration to better meet the needs of their different stakeholders.